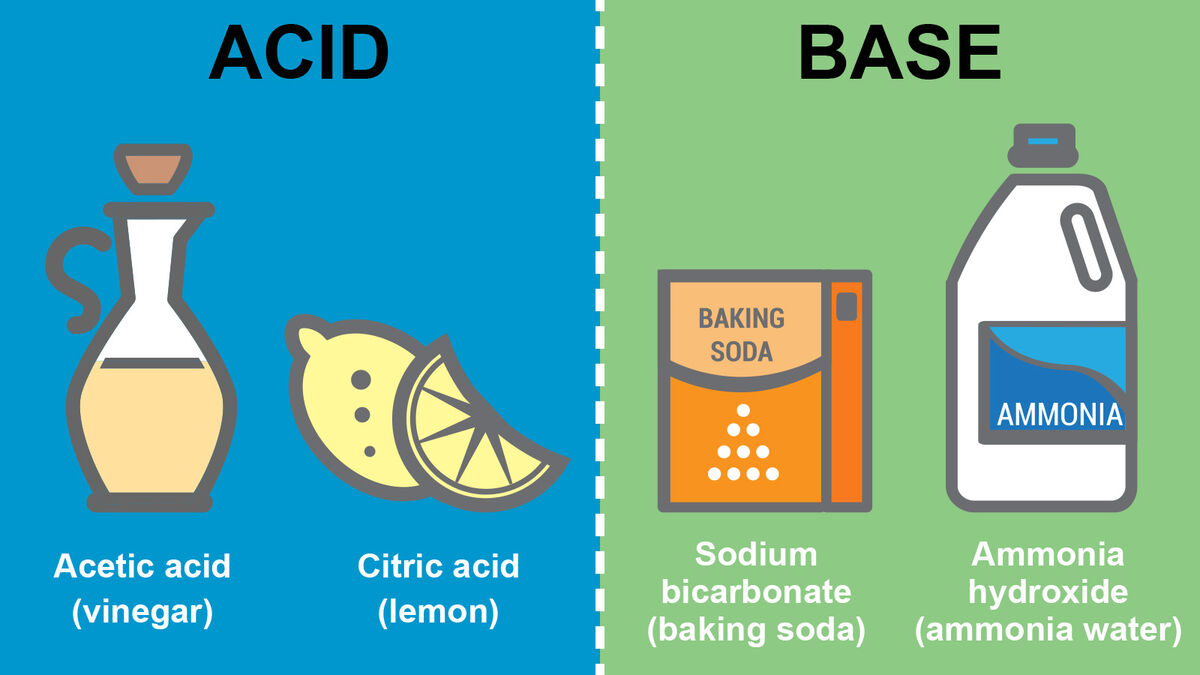
What Is Definition Of Base In Chemistry . Aqueous solutions of bases are also electrolytes. In chemistry, they are known for turning red litmus paper blue and are. Arrhenius bases, brønsted bases, and lewis bases. in chemistry, there are three definitions in common use of the word base : in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. Bases have properties that mostly contrast with those of acids. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. bases are substances that taste bitter and feel slippery when touched. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Bases can be either strong or weak, just. the earliest definition of acids and bases is arrhenius's definition which states that:
from www.yourdictionary.com
in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. bases are substances that taste bitter and feel slippery when touched. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just. In chemistry, they are known for turning red litmus paper blue and are. Bases have properties that mostly contrast with those of acids. the earliest definition of acids and bases is arrhenius's definition which states that:
Difference Between Acids and Bases Key Properties YourDictionary
What Is Definition Of Base In Chemistry In chemistry, they are known for turning red litmus paper blue and are. Bases can be either strong or weak, just. bases are substances that taste bitter and feel slippery when touched. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. Aqueous solutions of bases are also electrolytes. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. Arrhenius bases, brønsted bases, and lewis bases. Bases have properties that mostly contrast with those of acids. In chemistry, they are known for turning red litmus paper blue and are. the earliest definition of acids and bases is arrhenius's definition which states that: base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. in chemistry, there are three definitions in common use of the word base :
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is Definition Of Base In Chemistry In chemistry, they are known for turning red litmus paper blue and are. Bases have properties that mostly contrast with those of acids. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. bases are substances that taste bitter and feel slippery when touched. the. What Is Definition Of Base In Chemistry.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Is Definition Of Base In Chemistry In chemistry, they are known for turning red litmus paper blue and are. the earliest definition of acids and bases is arrhenius's definition which states that: bases are substances that taste bitter and feel slippery when touched. Bases can be either strong or weak, just. base, in chemistry, any substance that in water solution is slippery to. What Is Definition Of Base In Chemistry.
From www.sliderbase.com
Acid/Base definitions What Is Definition Of Base In Chemistry Arrhenius bases, brønsted bases, and lewis bases. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. bases are substances that taste bitter and feel slippery when touched. the earliest definition of acids and bases is arrhenius's definition which states that: Bases have properties that. What Is Definition Of Base In Chemistry.
From www.neatoshop.com
All Your Base (Chemistry) What Is Definition Of Base In Chemistry bases are substances that taste bitter and feel slippery when touched. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. Bases have properties that mostly contrast with those of acids. Arrhenius bases, brønsted bases, and lewis bases. in chemistry, there are three definitions in common use of the word base. What Is Definition Of Base In Chemistry.
From www.exampleslab.com
20 Examples of Chemical Bases Examples Lab What Is Definition Of Base In Chemistry Aqueous solutions of bases are also electrolytes. In chemistry, they are known for turning red litmus paper blue and are. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. Arrhenius bases, brønsted bases, and lewis bases. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide. What Is Definition Of Base In Chemistry.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free What Is Definition Of Base In Chemistry in chemistry, there are three definitions in common use of the word base : in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Arrhenius bases, brønsted bases, and lewis bases. An acid is a substance that forms hydrogen ions h + when dissolved in water,. What Is Definition Of Base In Chemistry.
From www.dtodoblog.com
Acids and Bases Chemistry D to Do blog What Is Definition Of Base In Chemistry Aqueous solutions of bases are also electrolytes. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. In chemistry, they are known for turning red litmus. What Is Definition Of Base In Chemistry.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary What Is Definition Of Base In Chemistry Bases can be either strong or weak, just. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. the earliest definition of acids and bases is arrhenius's definition which states that:. What Is Definition Of Base In Chemistry.
From studylib.net
Acids and Bases What Is Definition Of Base In Chemistry Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. Bases can be either strong or weak, just. in. What Is Definition Of Base In Chemistry.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2789112 What Is Definition Of Base In Chemistry bases are substances that taste bitter and feel slippery when touched. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. base, in chemistry, any substance that in. What Is Definition Of Base In Chemistry.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo What Is Definition Of Base In Chemistry Arrhenius bases, brønsted bases, and lewis bases. in chemistry, there are three definitions in common use of the word base : In chemistry, they are known for turning red litmus paper blue and are. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. base, in chemistry, any substance that in. What Is Definition Of Base In Chemistry.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1383826 What Is Definition Of Base In Chemistry bases are substances that taste bitter and feel slippery when touched. Bases can be either strong or weak, just. In chemistry, they are known for turning red litmus paper blue and are. Bases have properties that mostly contrast with those of acids. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter,. What Is Definition Of Base In Chemistry.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 What Is Definition Of Base In Chemistry In chemistry, they are known for turning red litmus paper blue and are. in chemistry, there are three definitions in common use of the word base : base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. Bases can be either strong or weak, just. the earliest. What Is Definition Of Base In Chemistry.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Is Definition Of Base In Chemistry in chemistry, there are three definitions in common use of the word base : In chemistry, they are known for turning red litmus paper blue and are. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Arrhenius bases, brønsted bases, and lewis bases. in chemistry, a base is a chemical. What Is Definition Of Base In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Is Definition Of Base In Chemistry in the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. in chemistry, there are three definitions in common use of the word base : Aqueous. What Is Definition Of Base In Chemistry.
From pediaa.com
Difference Between Acid and Base What Is Definition Of Base In Chemistry base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the colour of. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. the earliest definition of acids and bases is arrhenius's definition which states that: Aqueous solutions of bases are also electrolytes. An acid. What Is Definition Of Base In Chemistry.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions What Is Definition Of Base In Chemistry Arrhenius bases, brønsted bases, and lewis bases. In chemistry, they are known for turning red litmus paper blue and are. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. the earliest definition of acids and bases is arrhenius's definition which states that: in the field of chemistry, a ‘base’ can be. What Is Definition Of Base In Chemistry.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples What Is Definition Of Base In Chemistry An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. in chemistry, a base is a chemical species that donates electrons, accepts protons, or releases. in chemistry, there are three definitions in common use of the word base : In chemistry, they are known for turning red litmus paper blue and. What Is Definition Of Base In Chemistry.